S. Himmelstein | June 20, 2023
A carbonate-superstructured solid fuel cell (CSSFC) developed at Michigan Technological University offers greater fuel flexibility, higher durability and increased energy conversion efficiency at lower operating temperatures than other types of fuel cells.
An interface between the electrolyte and melted carbonate serves as an ultrafast channel for oxygen ion transfer. While the operating temperature of a conventional solid oxide fuel cell is usually 800° C or higher to spur ion transfer, the superstructured electrolyte in the CSSFC can provide fast ion transfer at 550° C or lower. The relatively low operating temperature offers high theoretical efficiency and lower cell fabrication costs.
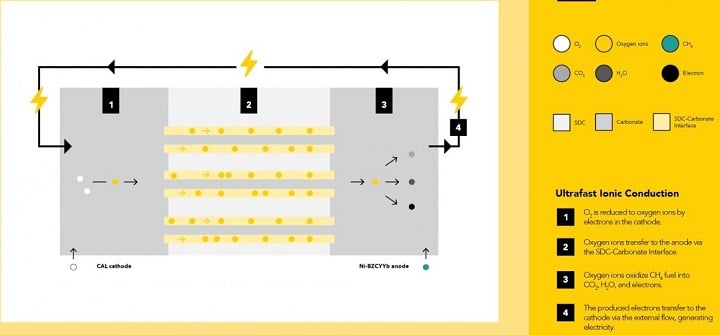 Schematic of ultrafast ionic conduction in the CSSFC. Source: Yun Hang Hu/Michigan Technological University
Schematic of ultrafast ionic conduction in the CSSFC. Source: Yun Hang Hu/Michigan Technological University
As explained in Proceedings of the National Academy of Sciences, in situ generation of superstructured carbonate in a porous samarium-doped ceria layer creates a unique electrolyte with ultrahigh ionic conductivity of 0.17 S/cm at 550° C. Tests demonstrated an unprecedentedly high open circuit voltage of 1.041 V and a high peak power density of 215 mW/cm2, which indicates no current leakage loss and high energy conversion efficiency. The continuous interface between molten carbonate and the solid ionic conductor provides a fast transfer channel for oxygen ions.
In addition to being powered by hydrogen, the fuel-flexible CSSFC can directly use methane or other hydrocarbon fuels. The researchers estimate that fuel efficiency of the device could reach 60%, far surpassing that of available solid oxide fuel cells.
