S. Himmelstein | July 19, 2022
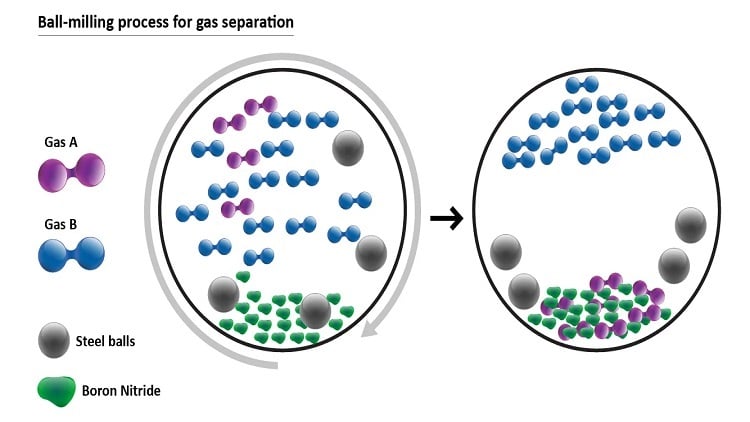 Schematic of mechanochemical separation of gases using ball milling. Source: Deakin University
Schematic of mechanochemical separation of gases using ball milling. Source: Deakin University
Pure hydrogen is either stored as a gas or as a cryogenic liquid, two options requiring considerable energy input. A novel process engineered by researchers in Australia represents a less energy intensive route to separate, store and transport huge amounts of gas safely, with no waste.
The approach mechanochemically traps and hold gases in powders via ball milling — a low-energy grinding process in which a cylinder containing steel balls is rotated, serving to crush the nanomaterial contained inside. Boron nitride powder is placed into a ball mill — a type of grinder containing small stainless-steel balls in a chamber — along with the gases that need to be separated. As the chamber rotates at a higher and higher speed, the collision of the balls with boron nitride powder and the wall of the chamber triggers a mechanochemical reaction resulting in gas being absorbed into the powder.
The ball-milling gas absorption process consumes 76.8 KJ/s to store and separate 1,000 liters of gas. The method consumes 90% less that used in the petroleum industry’s current separation process. Once absorbed into the powder, the gas can be transported safely and easily, and the material is heated in a vacuum to release the gas as needed.
The scheme has been demonstrated on a small scale with olefin and paraffin gases, separating about 2 L to 3 L of material. The researchers from Deakin University, Queensland University of Technology and RMIT University plan to scale up to a full pilot system with industry support and have submitted a provisional patent application for the technology described in Materials Today.
