S. Himmelstein | June 21, 2022
 Source: North Carolina State University
Source: North Carolina State University
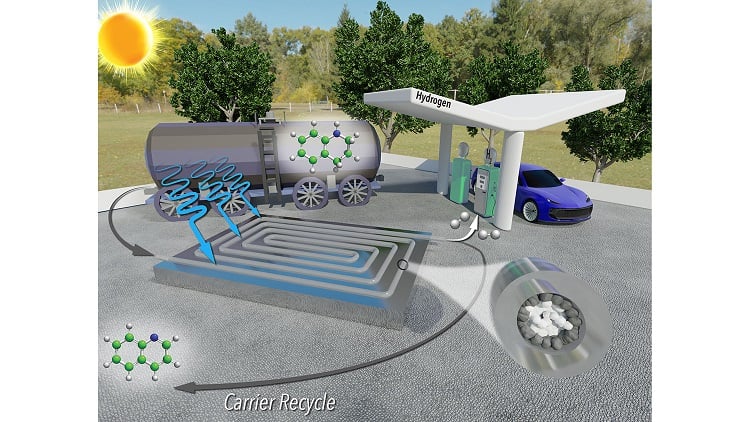
Use of a liquid carrier brings a high level of safety to the transport and storage of hydrogen but requires energy- and cost-intensive extraction at points of use, such as fueling stations. A photocatalytic process geared toward streamlining the extraction process has been engineered at North Carolina State University.
A provisional patent has been filed for the technology, which applies a reusable photocatalyst and sunlight to extract hydrogen gas from its liquid carrier more quickly and consumes less rhodium relative to available photocatalytic methods. Process byproducts are hydrogen gas and the liquid carrier itself, which can also be reused repeatedly.
A continuous-flow reactor is packed with micron-scale grains of titanium oxide coated with rhodium. These coated particles function as photoreactive catalysts that, when exposed to solar radiation, react with the liquid carrier to release hydrogen molecules as a gas. As only the outer grains of titanium oxide are coated with rhodium, the cost is considerably lower than that of a conventional batch reactor.
The system tested released 99% of the hydrogen molecules from the liquid carrier in three hours, eight times faster than conventional batch reactors, which require 24 hours to reach 99% yield.
The flow system described in ChemSusChem can run continuously for up to 72 hours before its efficiency decreases.
